SISTM
Statistics in systems biology and translationnal medicine


Objectives
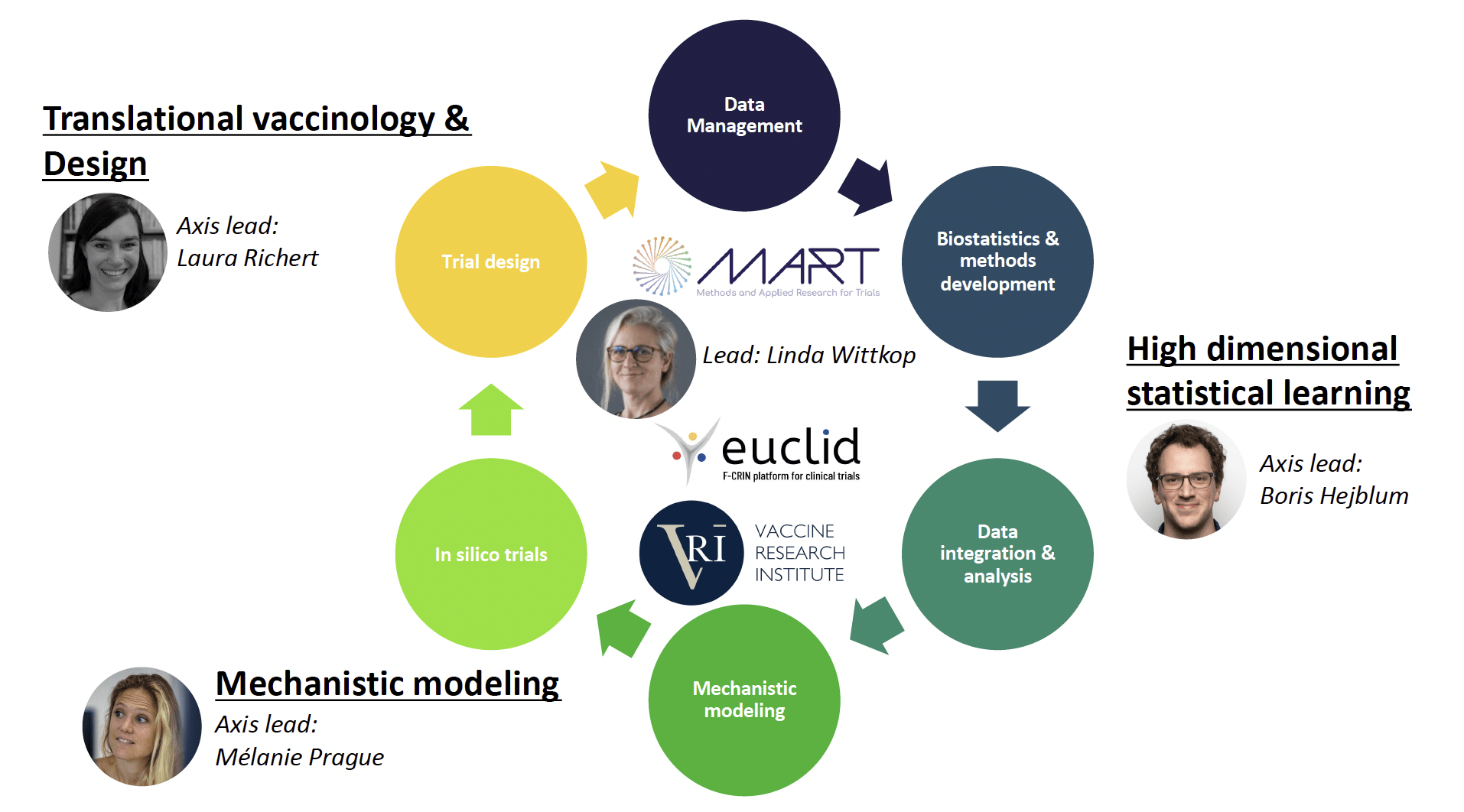
The two main objectives of the SISTM team are :
1) to accelerate the development of vaccines by analysing all the information available in early clinical trials and optimizing new trials
2) to develop new statistical methods to analyse and model large high-throughput data.
Research area
The relevant information is extracted from large omics data, and this signal is then incorporated into mechanistic models, thanks to prior biological knowledge, to estimate their parameters. Those models can then inform the optimal vaccine strategies to be evaluated in the next clinical trials. Such in silico trials, allowing for optimized clinical trial designs and personalized strategies, in turn generate new data that require and feed anew the above methodological developments.
Downloads
For Lasso Estimation of Conditional Logistic
Regression Models for matched case-control and case-crossover studies
2024 Key publications
Alexandre M, Prague M, Lhomme E, Lelievre J-D, Wittkop L, Richert L, Levy Y, Thiebaut R. Definition of Virological Endpoints Improving the Design of HIV Cure Strategies Using Analytical Antiretroviral Treatment Interruption. Clin Infect Dis. 2024;79(6):1447-57. https://doi.org/10.1093/cid/ciae235
Barry H, Lhomme E, Surenaud M, Nouctara M, Robinson C, Bockstal V, Valea I, Somda S, Tinto H, Meda N, Greenwood B, Thiebaut R, Lacabaratz C. Helminth exposure and immune response to the two-dose heterologous Ad26.ZEBOV, MVA-BN-Filo Ebola vaccine regimen. PLoS Negl Trop Dis. 2024;18(4):e0011500. https://doi.org/10.1371/journal.pntd.0011500
Ganser I, Buckeridge DL, Heffernan J, Prague M, Thiebaut R. Estimating the population effectiveness of interventions against COVID-19 in France: A modelling study. Epidemics. 2024;46:100744. https://doi.org/10.1016/j.epidem.2024.100744
Hejblum BP, Ba K, Thiebaut R, Agniel D. Neglecting the impact of normalization in semi-synthetic RNA-seq data simulations generates artificial false positives. Genome Biol. 2024;25(1):281. https://doi.org/10.1186/s13059-024-03231-9
Hivert B, Agniel D, Thiébaut R, Hejblum BP. Post-clustering difference testing: Valid inference and practical considerations with applications to ecological and biological data. Comput Stat Data Anal. 2024;193:107916. https://doi.org/10.1016/j.csda.2023.107916
Levy Y, Moog C, Wiedemann A, Launay O, Candotti F, Hardel L, Durand M, […], Thiebaut R, Pantaleo G, Lelievre J-D, Richert L. Safety and immunogenicity of CD40.HIVRI.Env, a dendritic cell-based HIV vaccine, in healthy HIV-uninfected adults: a first-in-human randomized, placebo-controlled, dose-escalation study (ANRS VRI06). eClinicalMedicine. 2024;77:102845. https://doi.org/10.1016/j.eclinm.2024.102845
Loubet P, Lelievre J-D, Francois A, Botelho-Nevers E, Chidiac C, Chirio D, Dubee V, Dussol B, Galtier F, Hessamfar M, […], Launay O, Wittkop L. Humoral response after mRNA COVID-19 primary vaccination and single booster dose in people living with HIV compared to controls: A French nationwide multicenter cohort study-ANRS0001s COV-POPART. Int J Infect Dis. 2024;146:107110. https://doi.org/10.1016/j.ijid.2024.107110
Savel H, Meyer-Losic F, Proust-Lima C, Richert L. Statistical classification of treatment responses in mouse clinical trials for stratified medicine in oncology drug discovery. Sci Rep. 2024;14(1):934. https://doi.org/10.1038/s41598-023-51055-7
Segalas C, Helmer C, Genuer R, Proust-Lima C. Functional Principal Component Analysis as an Alternative to Mixed-Effect Models for Describing Sparse Repeated Measures in Presence of Missing Data. Stat Med. 2024;43(26):4899-912. https://doi.org/10.1002/sim.10214
Valayer S, Alexandre M, Prague M, Beavogui AH, Doumbia S, Kieh M, Greenwood B, Leigh B, Poupelin M, […], Thiebaut R, Levy Y, Yazdanpanah Y, Richert L, Lhomme E. Evaluation of waning of IgG antibody responses after rVSVDeltaG-ZEBOV-GP and Ad26.ZEBOV, MVA-BN-Filo Ebola virus disease vaccines: a modelling study from the PREVAC randomized trial. Emerg Microbes Infect. 2024;14(1):2432353. https://doi.org/10.1080/22221751.2024.2432353
Last News


Launch of CAP Digital Health within the framework of the call for expressions of interest "Skills and Future Professions"
Events
Covid-19 : Etude de l'efficacité des mesures instaurées contre le virus en France
Press
The BPH SISTM team is accredited by both Inria & Inserm
Team
More than 3 M€ for our project UB 2030 CAP Digital Health labellized by the call AMI CMA "Skills and future professions" of the 2030 France national plan
AwardsMembers
Avalos FernandezMarta
Associate Professor in BiostatisticsBAKALIDOU
Etudiant en thèseBoespflugNicolas
Ingénieur d'étudesClaironQuentin
Chargé de Recherche INRIAColajanniAntonin
Etudiant en ThèseDarmignySandrine
Assistante administrativeEL HAJJZeinab
Ingénieure de rechercheEl KarchiJad
Ingénieur R&DFALLETSARA
Ingénieur d'étudesFerteThomas
Etudiant en thèseGABAUTAuriane
Étudiante en thèseGanserIris
Etudiante en thèseGenuerRobin
Maître de ConférencesGounaDaniela
DoctoranteGUERRA ADAMESAriel
Doctoral StudentHejblumBoris
Chargé de Recherche INSERMHivertBenjamin
Post-doctorant / Post-doctoral fellowHuchonMélanie
Ingénieure d'études biostatisticienneHUGHESArthur
Etudiant en thèseLAVALQuentin
Ingénieur d'étudesLhommeEdouard
MD, PhD - MCU-PH Santé PubliqueLiZhe
DoctoranteNAFTI MAHERZIMyriam
Data ManagerOttaviAnton
Coordinateur de Projets InternationauxPalAnnesh
DoctorantPragueMélanie
Chargé de Recherche InriaREMIATJustine
Doctorante en Santé Publique - BiostatistiqueReynéBastien
PostdocRichertLaura
MD, PhD - PU-PH Santé PubliqueRuizAnne-Andrée
Ingénieure d'études BiostatisticienneSAVELHelene
Etudiante en thèseSegalasCorentin
Research Fellow in statisticsThiebautRodolphe
Directeur d'équipeWittkopLinda
MD PhD - PU-PH Santé Publique