ANRS VRI06 Phase I clinical trial for a preventive HIV vaccine
RetourMore than 40 years after the discovery of HIV, the development of a vaccine remains essential to control the epidemic. There are currently no vaccine candidates in phase III trials, all of which have been stopped due to lack of efficacy.
Our team is part of a consortium developing new vaccine candidates based on new technologies.

And researchers from the Vaccine Research Institute (VRI) of Inserm and the University of Paris-Est Créteil (Unit 955 Institut Mondor de recherche biomédicale), the CHUV in Lausanne and the BPH have obtained encouraging results for an innovative preventive HIV vaccine candidate in a study conducted in France and Switzerland.
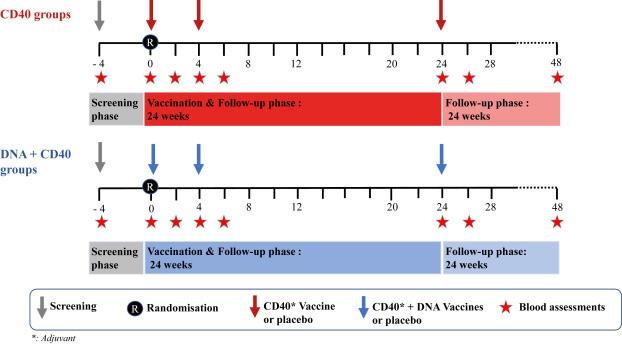
This new phase I ANRS VRI06 trial, conducted by Inserm, ANRS MIE and VRI, shows that this vaccine, called CD40.HIVRI.Env, is safe and capable of inducing a rapid and durable humoral and cellular immune response against HIV.
This is the first human trial of this vaccine, which consists of a monoclonal antibody fused to the HIV envelope that specifically targets the CD40 molecule expressed on the surface of dendritic cells, the cells involved in mounting protective immune responses.
Participants, 72 HIV-negative volunteers, were monitored for safety and immune responses.
The study concludes that the CD40.HIVRI.Env vaccine, with or without DNA HIV-PT123, is safe and effective in inducing sustained cellular and humoral immune responses, suggesting its potential utility in prime-boost vaccination strategies against HIV.
More information :
Press release :
https://www.u-bordeaux.fr/application/files/5517/2787/7878/CP_CD40.HIVRI.Env_270924_Fr.pdf
Link to the publication :
https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(24)00424-3/fulltext


